Reliability and validity of the EasyForce® dynamometer compared to handheld dynamometry for isometric strength testing of the upper and lower extremities
Authors: John Karl (PT, DPT), Birendra Dewan (PT, PhD), Michael Masaracchio (PT, PhD), Kaitlin Kirker (PT, DPT)
Department of Physical Therapy, Long Island University, 1 University Plaza, Brooklyn, NY
Keywords: Force Production, Lafayette Manual Muscle Tester, Strength Assessment, Muscle Testing
Abstract
A new pull-type dynamometer, the EasyForce®, may offer clinical advantages for measuring strength. The purpose of the current study was to assess the intra-rater reliability of the EasyForce® and its concurrent validity compared to the Lafayette Manual Muscle Tester in healthy individuals.
Methods
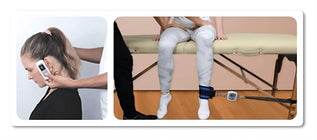
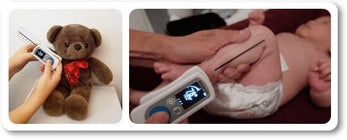
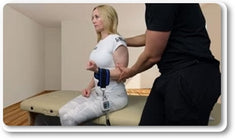
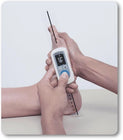
Maximal voluntary isometric contraction was tested using the Lafayette Manual Muscle Tester and the EasyForce® to explore the association between the two instruments. Measurements of the shoulder girdle and knee were performed on healthy, active individuals recruited from the undergraduate and graduate student population on two separate days, with 48–72 hours between sessions and at the same time of day for each participant.
Results
Thirty participants (age 29.97 ± 3.25 years) were enrolled in the study. Intraclass correlation coefficients for the EasyForce® ranged from 0.87–0.98 for the shoulder and 0.80–0.81 for the knee. Assessment of concurrent validity demonstrated strong correlations between the EasyForce® and Lafayette dynamometers for all measurements in both sessions, which were statistically significant (τ_b = 0.45 to 0.78, p < .001). Wilcoxon signed-rank tests showed statistically significant differences between devices during both sessions (p < 0.001 to p = 0.02).

Clinical Relevance
Based on the results of this study, clinicians should consistently use the same dynamometer, regardless of type, across treatment sessions to measure strength changes.
Conclusion
The EasyForce® is a reliable and valid tool to measure the strength of the upper and lower extremities.